Our Research
Our research group focuses on the design and development of novel therapeutic agents through the integration of medicinal chemistry, chemical biology, and advance computatioanl drug discovery approaches. We work on various projects targeting different disease areas, with a particular emphasis on cancer and infectious diseases.
Antimicrobial Drug Development
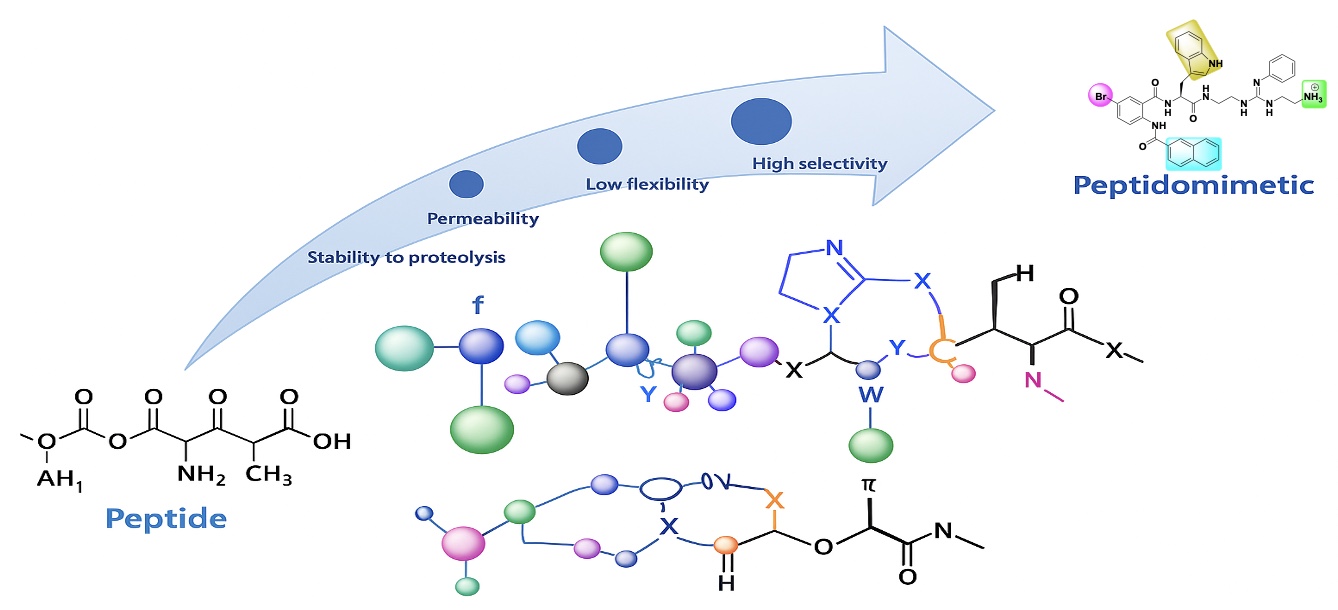
1. Antimicrobial Peptide Mimics
Our group is developing novel antimicrobial peptide mimics to combat drug-resistant bacteria. This research includes:
- Design and synthesis of peptide mimics
- Structure-activity relationship studies
- Mechanism of action studies
- In vitro and in vivo evaluation
2. Antimicrobial Hydrogel
We are investigating the antimicrobial properties of hydrogel-based compounds, focusing on:
- Development of novel hydrogel-based antimicrobial agents
- Mechanism of antimicrobial action
- Synergistic effects with existing antibiotics
- Safety and efficacy studies
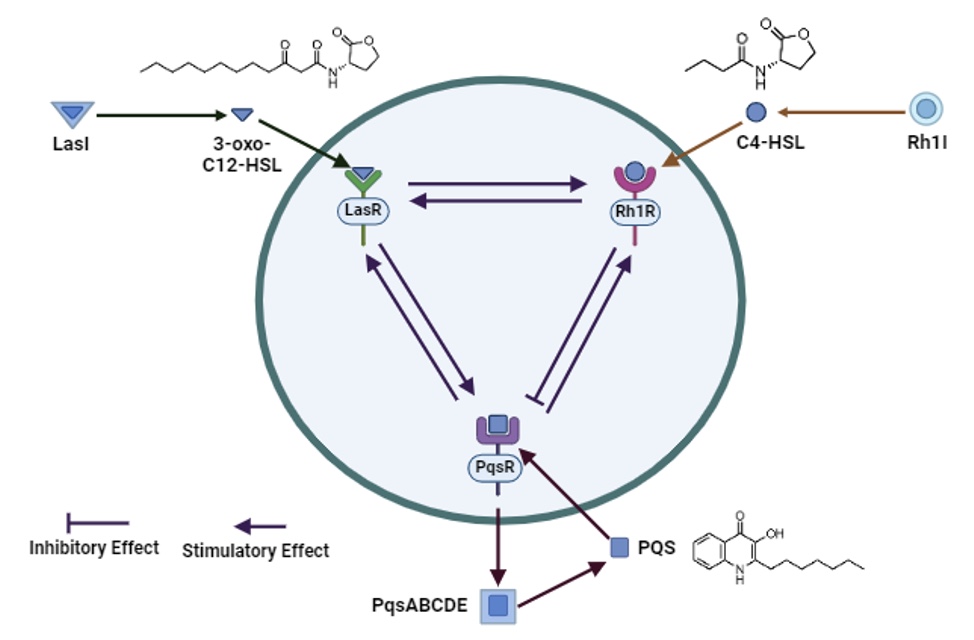
3. Quorum Sensing Inhibitors
Our research on quorum sensing inhibitors aims to disrupt bacterial communication, including:
- Design of novel quorum sensing inhibitors
- Target identification and validation
- Biofilm disruption studies
- In vitro and in vivo evaluation
Cancer Drug Discovery
1. Targeting MYCN with New Scaffolds for Anticancer Discovery
In collaboration with Dr Belamy Cheung and Prof Glenn Marshall (CCIA, UNSW), we are developing novel therapeutic approaches for neuroblastoma (NB), the most common extracranial solid tumour in early childhood. Our research focuses on:
- Targeting MYCN oncogene amplification and overexpression
- Development of novel inhibitors for ubiquitin specific protease 5 (USP5)
- Restoration of cell cycle checkpoint control and induction of apoptosis
- In vitro and in vivo evaluation of new therapeutic scaffolds
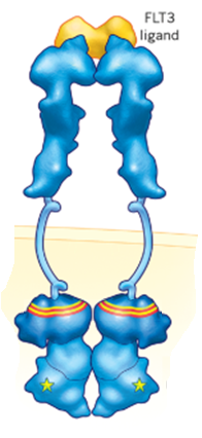
2. Design And Synthesis of Novel Inhibitors Targeting Acute Myeloid Leukemia
(in collaboration with Dr Daniel Wenholz UNSW) Acute Myeloid Leukemia (AML) is a blood cancer (leukaemia) that represents ~40% of all new adult-onset leukemias in Australia. It is characterised by the overproduction of abnormal myeloblasts in the bone marrow, preventing healthy myeloblast, platelet and erythrocyte production. FMS-like tyrosine kinase 3 (FLT3) is a class III tyrosine kinase receptor involved in the regulation of hematopoietic cell differentiation, survival and proliferation. FLT3 mutations are among the most frequently identified mutations involved in leukaemia development and occur in approximately 28% of AML patients. Mutations of FLT3 have been associated with a poor prognosis, specifically adverse disease features, poor survival and a reduced rate of remission. A derivative of phenoxodiol has been identified as a screening hit compound for inhibition against FLT3 mutants. The overall aim of this project is to investigate the structure activity relationship on FLT3 through the synthesis of a library of novel analogues.
Computational Drug Discovery
1. AI-Driven Drug Design
Amid the growing global AMR crisis, there is an urgent need for efficient methods to discover novel antimicrobial peptides. Integrating computational tools such as virtual screening and mining of genomic data offers a scalable and rapid alternative to traditional approaches. on this collaborative project harnesses advances in computational methods, including machine learning, and deep learning, to enable large scale and accurate screening of AMPs with fewer false positives and the ability to predict activity prior to experimental testing.

2. Molecular Simulation & Dynamics
This project involves the use of molecular docking against key target receptors, integrated with molecular dynamics (MD) and steered MD simulations. This interdisciplinary approach accelerates the drug discovery process and aligns with green chemistry principles by minimizing laboratory waste. It facilitates the development of more effective and selective antimicrobial agents, contributing to the advancement of next-generation therapeutics with enhanced safety and environmental sustainability
Development of Scalable Synthetic Strategies
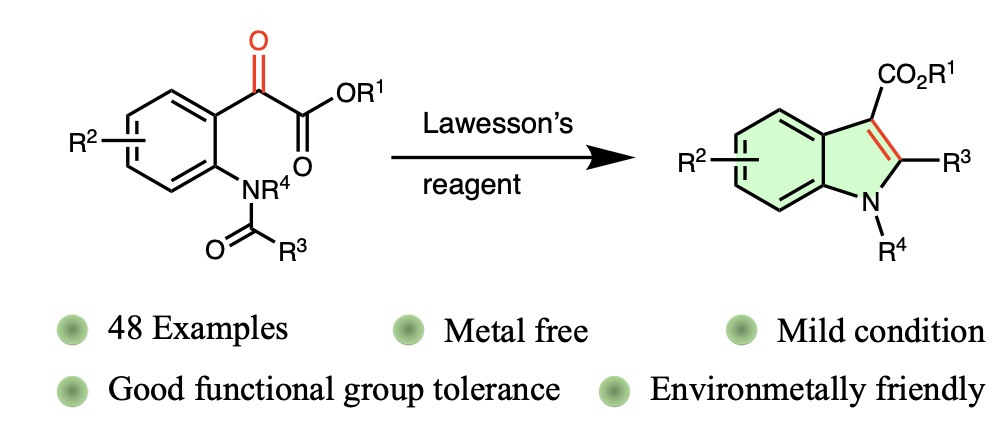
Synthesis of Indoles through C2−C3 Bond Formation Using Lawesson's Reagent
We have developed an efficient, metal-free synthetic route for the construction of functionalized indoles through C2–C3 bond formation, mediated by Lawesson's reagent. This versatile methodology employs readily available amidophenylglyoxylic esters and exhibits broad substrate tolerance, yielding a wide range of 2,3-disubstituted indoles. The process is scalable to gram quantities and facilitates diverse downstream modifications, underscoring its significant utility in organic synthesis.
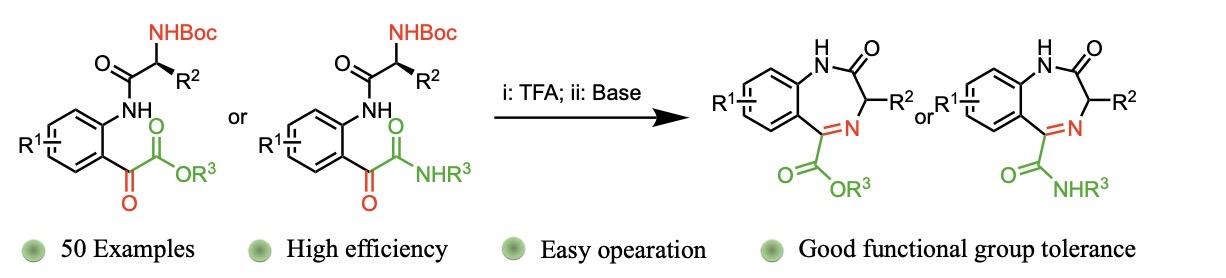
Synthesis of 1,4-benzodiazepin-2-ones from isatins
We have developed an effective synthetic strategy for the construction of 1,4-benzodiazepin-2-ones, a crucial class of compounds in medicinal chemistry with broad therapeutic applications. Our method provides a versatile approach to synthesize derivatives bearing diverse ester or amide functionalities at the C5 position, addressing a previously challenging area. This high-yielding protocol demonstrates excellent substrate tolerance, enabling the efficient preparation of over 50 novel benzodiazepine analogues. Furthermore, the process is scalable to gram quantities, showcasing its potential for practical applications in drug discovery and development.